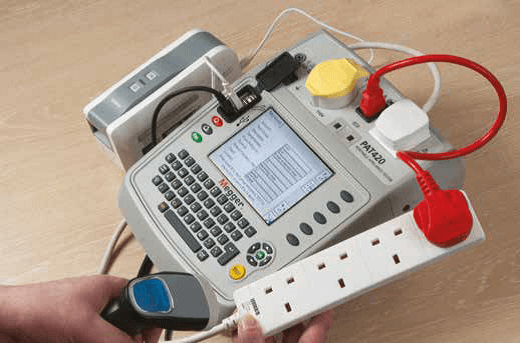
The categories of appliance, which should be considered for PAT testing:-
- Fixed appliances.
- Stationary appliances.
- IT appliances.
- Moveable appliances.
- Portable appliances.
- Cables and chargerss.
- Hand Held appliances.
- Extension lead.
PAT test an essential safety measure for your businesses to achieve the following:
- PAT testing is the way of maintaining electrical appliances and it:
- Reduces the risk of the fires.
- Prevent users getting an electrical shock.
- Is a legal requirement in the work place.
- One condition for insurance companies .
- Part of electricity at work regulation.
- Employers, school and hotel owners must ensure work and environment equipment is suitable for the use and safe.
For more information, please contact us: info@gtsco-uae.com
A well-executed cleanroom testing validation and certification program is essential to proper cleanroom maintenance and operation and regulatory compliance. GTSCO using their Partners long experience in cleanroom performance testing offers comprehensive clean room services and solutions.
Cleanroom test protocols and report documentation can be customized to customer specifications.
Primary cleanroom tests include:
- Airflow volume/velocity profiling.
- Room air exchange rates.
- HEPA filter integrity testing.
- Non-viable particulate monitoring.
- Room pressurization monitoring.
- Temperature and humidity monitoring.
Optional tests include:
- Airflow visualization testing.
- Viable environmental monitoring (EM) including airborne and surface microbial.
- Enumeration and identification.
- Lights testing.
- Sound testing.
- Vibration testing.
- Other cleanroom tests as per IEST and/or ISO.
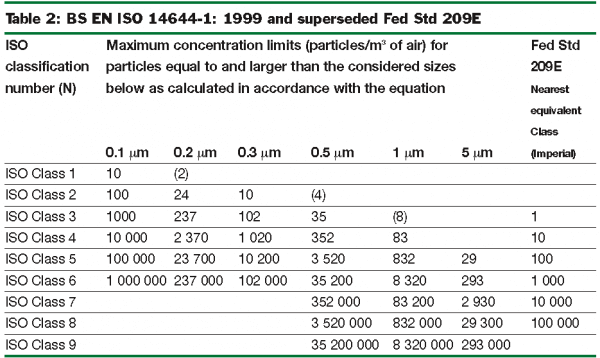
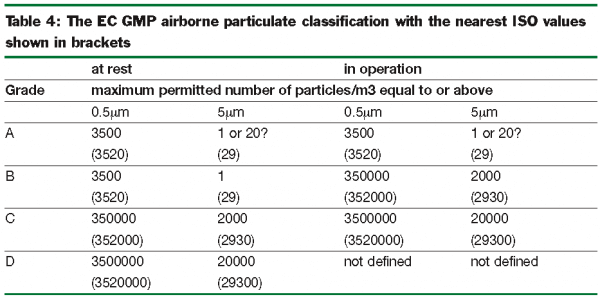
Cleanroom validation is provided by conducting a series of tests to qualify if a controlled environment is performing in accordance with process requirements and the applicable regulatory guidelines, such as ISO 14644-1:2015 ,US Fed 209E ,EU GMP Annex 1.
With each service GTSCO offers:
- Trained and accredited technical service of the highest qualitys.
- Customized and quality-reviewed report documentation tailored to your needs.
- Management of your equipment inventory and service records.
For more information, please contact us: info@gtsco-uae.com
To gauge the effectiveness of a cleaning program, facilities and healthcare rely on visual inspection as a cleaning monitoring method. Although easy to implement, visual inspection has been shown to be inadequate for ensuring proper cleaning has been performed.
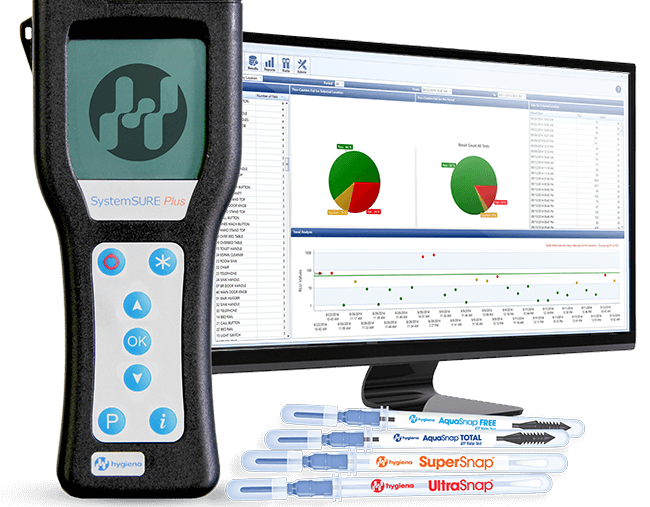
Our Service to provide ATP tests part of our cleaning service that enable customers to check for cleanliness on the microscopic level in a quick, cost-effective, and objective manner. These tests are performed in seconds and enable immediate remediation if needed.
Adenosine triphosphate (ATP) is an enzyme that is present in all living cells, and an ATP monitoring system can detect the amount of organic matter that remains after cleaning an environmental surface, a medical device or a surgical instrument. Hospitals are using ATP-based sanitation monitoring systems to detect and measure ATP on surfaces as a method of ensuring the effectiveness of their facilities sanitation efforts. The amount of ATP detected, and where this ATP was detected, indicates areas and items in the healthcare setting that may need to be recleaned, and the possible need for improvement in a healthcare facilitys cleaning protocols.
Why you have to do ATP test:
- Instantly assess the cleanliness of production surfaces, allowing immediate corrective action to be taken before production begins
- Reduce the use of conventional microbiological testing methods that are slow, labor intensive and costly
- Optimize cleaning chemicals, equipment and labor so that the plant can maintain a high cleanliness level without an excessive amount of waste
- Standardize the level of cleanliness and verify efforts of sanitation personnel
- Increase product quality and extend shelf-life by preventing product residues and other contamination from coming in contact with new product
- Record and track test results to identify problem areas, make improvements and show due diligence and compliance with HACCP, Sanitation Standard Operating Procedures (SSOPs) and industry regulations
For more information, please contact us: info@gtsco-uae.com


 Data center Solutions
Data center Solutions Cleanroom Solutions
Cleanroom Solutions Swimming Pool
Swimming Pool  Kitchen hood and duct
Kitchen hood and duct